TMS THERAPY
- Minds Over Matter
- TMS THERAPY

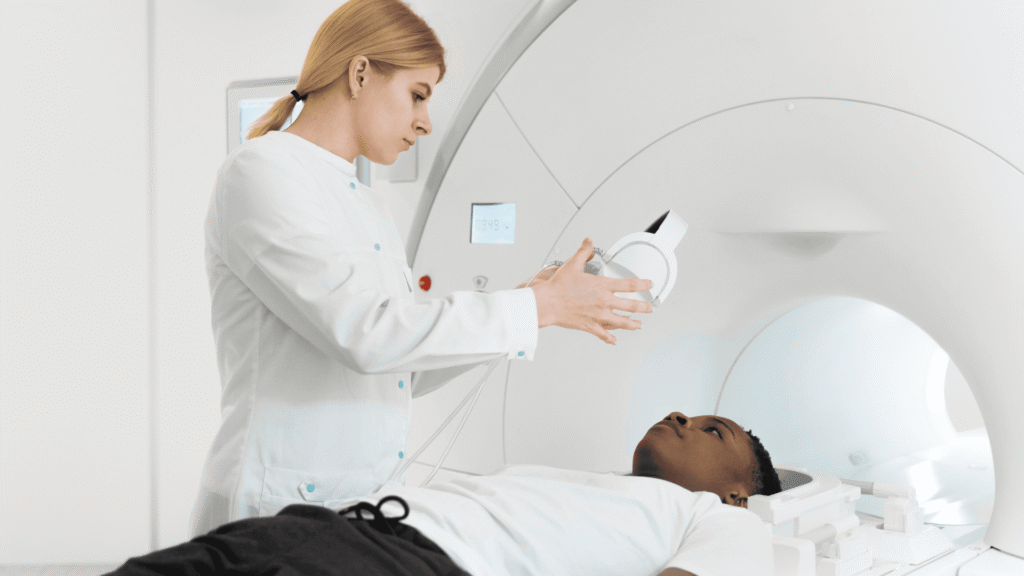
TMS Therapy
At Minds Over Matter, we proudly offer Transcranial Magnetic Stimulation (TMS) Therapy — a safe, non-invasive treatment that uses magnetic pulses to stimulate areas of the brain linked to mood regulation. TMS is FDA-approved and clinically proven to help individuals who struggle with depression, anxiety, or other mood disorders — especially when traditional treatments haven’t been effective.
Our TMS program is personalized, compassionate, and rooted in cutting-edge mental health science. Whether you’re exploring alternatives to medication or looking for a new path forward, TMS Therapy could be the breakthrough you’ve been waiting for.
- Non-Invasive & Drug-Free
- Clinically Proven Results
- Precision-Targeted Treatment
- Improves Quality of Life

Where Can I Get Some?
How long does each TMS session take?
Each session typically lasts around 20–30 minutes. You can return to your normal activities immediately afterward — no downtime required.
Who is a good candidate for TMS therapy?
TMS is ideal for individuals with treatment-resistant depression or those seeking alternatives to medication. A full evaluation with our team will determine eligibility.
Is TMS Therapy safe?
Yes, TMS is FDA-approved and considered very safe. Side effects are minimal and usually include mild scalp discomfort or headache that resolves quickly.

Get In Touch With Us
Get in Touch With
Minds Over Matter
Local:(954) 947-3607
+(954) 947-3607
Email: Info@mombhc.com
Info@mombhc
Address: 1280 S Powerline Rd, Pompano Beach, FL 33069
1280 S Powerline Rd, Pompano Beach, FL 33069

